Update on COVID-19 Vaccines [] BeWell San Miguel
News Category: News, COVID 19, and Health/Fitness
-
Published January 16. 2021
Ellie Burleigh (MPH, Phd)
This update includes new information and guidance from US and Mexican sources regarding vaccine approvals, vaccine availability, answers to FAQs, descriptions of vaccine products and their distribution, and plans for additional vaccines.
Which vaccines have been approved in the US and Mexico so far?
US: Two vaccines have been approved for emergency use in the United States: The Pfizer-BioNTech vaccine and the Moderna vaccine. Both are mRNA vaccines. Both require two doses, spaced three or four weeks apart. The first dose, the primary dose, provides some immunity during the time between the two doses but it is the second dose that raises immunity levels to an astonishing 94-95%.
Mexico: To date, Mexico has approved the Pfizer-BioNTech vaccine and the AstraZeneca vaccine (https://elpais.com/mexico/
2021-01-05/mexico-aprueba-la- vacuna-contra-el-coronavirus- de-oxford-y-astrazeneca.html). The Pfizer-BioNTech vaccine is an mRNA vaccine. The AstraZeneca vaccine is not. It is an adeno-virus vectored vaccine. Both of these vaccines require two doses, spaced about a month apart. Detail on each of the approved vaccines is provided be low. These are the only vaccines approved in each country so far. In spite of rumors of other vaccine sources, Mexico has not approved any other vaccine to date.
Vaccination Phases
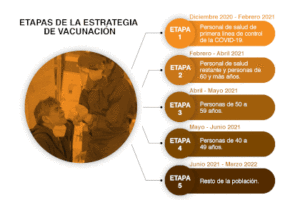 Mexico: In Phase I: Vaccination of health personnel – January, 2021 begin vaccinating people against COVID-19 nationally. On that same date, shipments began arriving of over 400,000 doses of the Pfizer-BioNTech vaccine. The objective is to have vaccinated all medical and health personnel who work directly with persons with COVID-19 in the 1,015 “COVID-19 hospitals” in the country by the end of January. Each person is to receive the recommended two shots 3 weeks apart.
Mexico: In Phase I: Vaccination of health personnel – January, 2021 begin vaccinating people against COVID-19 nationally. On that same date, shipments began arriving of over 400,000 doses of the Pfizer-BioNTech vaccine. The objective is to have vaccinated all medical and health personnel who work directly with persons with COVID-19 in the 1,015 “COVID-19 hospitals” in the country by the end of January. Each person is to receive the recommended two shots 3 weeks apart.The next step beginning in February will be to vaccinate the elderly, beginning with those over 80, 70-79, 60-69, etc., until all those over 60 are vaccinated. Dr. Lopez-Gatell, SubSecretary of Health, has been clear that brigades will begin working first in the most remote areas of the country once they have completed the vaccination of all health personnel.
On January 6, Dr. Lopez Gatell, explained that vaccinations for the elderly will be given by brigades made up of trained public servants and volunteers. Each brigade will be set up in one of 10,000 Integrated Centers, to be located in the geographical center of 280,000 small, disperse communities in the country. Centers will be located where the elderly are accustomed to receive their pensions (a school, rural medical unit, a plaza, etc.). Each Center will vaccinate 300 elderly adults each week. In this way, Mexico will immunize a total of 3 million elderly persons in remote areas. Once the Centers are installed, the persons will receive their vaccine when they receive their bi-monthly pension. If the person does not come, a brigadista will go to their home. A group of authorities from the communities surrounding each Center will send a report of the vaccination campaign to the capital of each state.
Following this, vaccinations will proceed in the 2,500 municipalities or municipal heads, and then the largest cities. When the same brigades reach the large cities, they will work together, with each brigade responsible for vaccinating 300 persons a week. Once all of the elderly are vaccinated, vaccination will continue with persons under 60 who have chronic diseases, and with teachers under 60 years of age.
Guanajuato
On January 11, 2021, The Secretary of Health of Guanajuato announced that the State is ready to begin vaccinating its 22,425 health professionals against COVID-19. It will take up to 18 months to immunize over 70% of the population of Guanajuato. Guanajuato has the ability to vaccinate large numbers of people, and will follow the national plan. https://boletines.guanajuato.
gob.mx/2021/01/11/guanajuato- esta-preparado-para-iniciar- este-miercoles-la-vacunacion- contra-el-coronavirus/ Those excluded will include children under 16, and pregnant women.
USA
The US has given Emergency approval (EUA) to the Pfizer and the Moderna vaccines and has begun vaccination in all states. The vaccination situation for each state can be found online. According to CDC, as of January 11, 2021, nearly 9 million initial doses of the two approved vaccines have been administered. Each state is administering its own program. If you decide to get vaccinated in the US, check your state’s guidance, and register if possible.
The problems with the US vaccination roll-out have been due, primarily, to a confusion in lines of command and responsibility. Initially, states were led to believe that the federal government would be coordinating the entire program, with the help of the military. Later, this proved not to be the case, and the responsibility was unexpectedly given to each of the states. Very few had prepared the necessary personnel and cold chain to handle a massive vaccination campaign. Most states have tried to rise to the challenge, developing plans and approaches, but many still struggle. Hopefully this will straighten itself out soon.
Additional COVID-19 vaccines are being considered in the US and expected to be submitted to the FDA for EUA approval in 2021. Large-scale (Phase 3) clinical trials are in progress for additional COVID-19 vaccines in the US.
. Janssen/Johnson and Johnson’s COVID-19 vaccine
. AstraZeneca
And that’s it for now! I hope this has been useful.
Ellie Burleigh (MPH, Phd)
-

Leave a Reply